The Transgenic Fly
 Molecular biology techniques such as PCR and restriction digests are employed to generate myosin transgenes that are injected into Drosophila embryos to create transgenic fly lines. The transgene might be designed to express myosin with a mutation that causes muscle or heart diseases in humans.
Molecular biology techniques such as PCR and restriction digests are employed to generate myosin transgenes that are injected into Drosophila embryos to create transgenic fly lines. The transgene might be designed to express myosin with a mutation that causes muscle or heart diseases in humans.
Protein Engineering
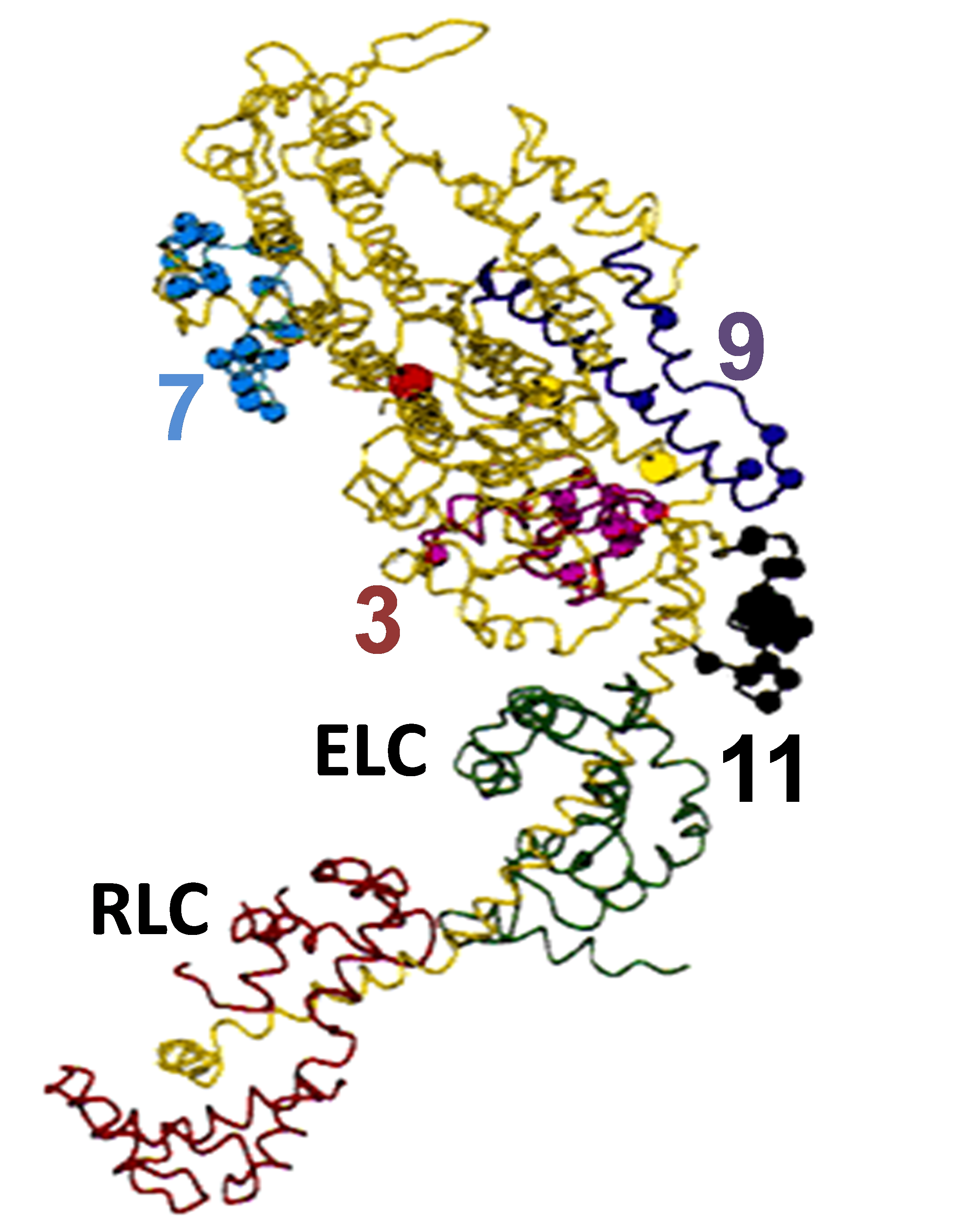 To determine the role of various domains of proteins to their function, we can mutate specific amino acids or exchange entire domains between protein isoforms. Two specific domains being tested are the converter and the relay domain of muscle myosin. Using the transgenic fly as a vessel for expressing engineered proteins, we can observe the functional effects on muscle and heart performance.
To determine the role of various domains of proteins to their function, we can mutate specific amino acids or exchange entire domains between protein isoforms. Two specific domains being tested are the converter and the relay domain of muscle myosin. Using the transgenic fly as a vessel for expressing engineered proteins, we can observe the functional effects on muscle and heart performance.
Muscle Mechanics
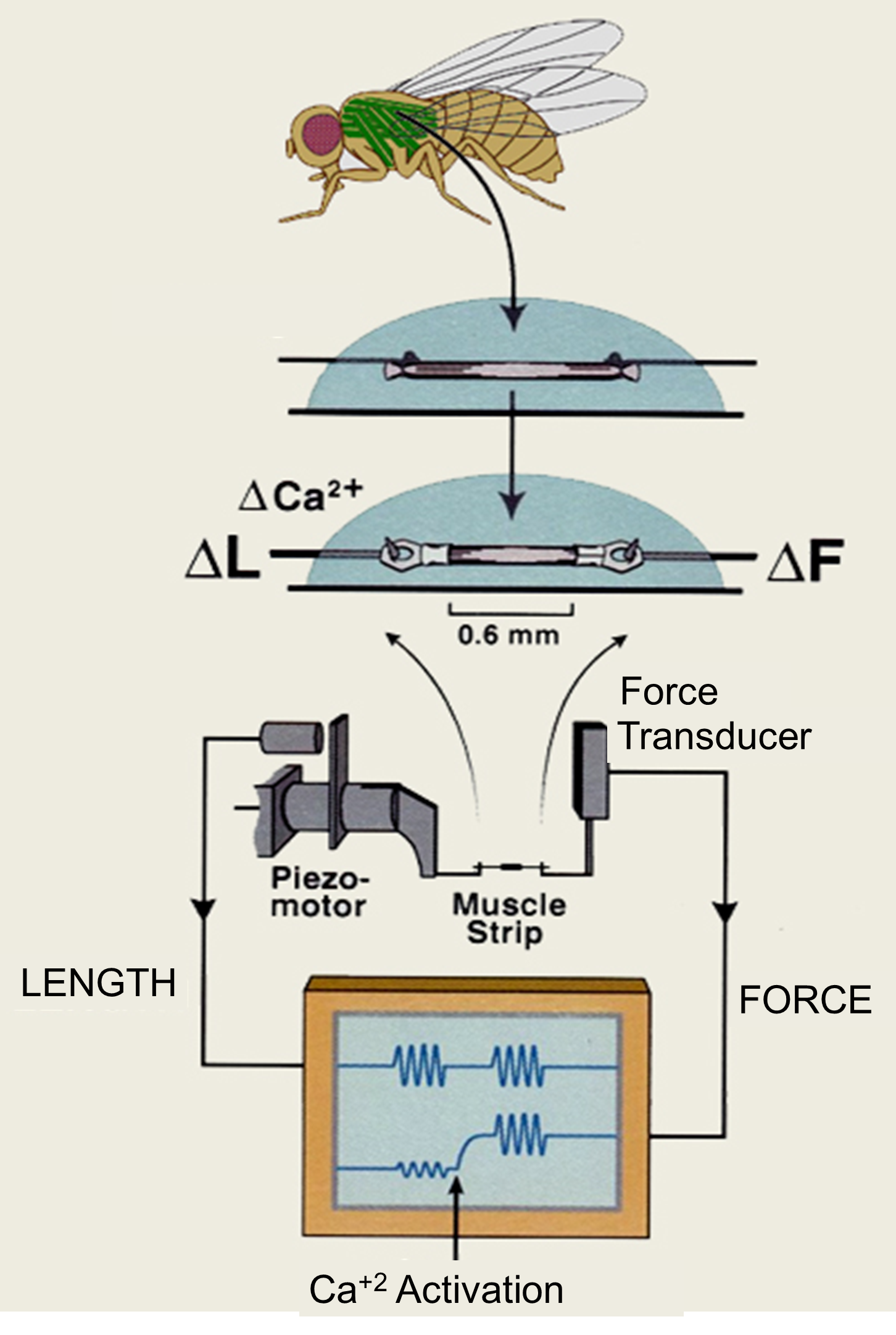 We evaluate muscle mechanical properties using the skinned fiber technique. The mechanics apparatus includes a pair of hooks, one attached to a force transducer and the other to a high-speed length motor. The muscle fiber is mounted onto the hooks and lowered into a solution chamber. By varying the concentrations of the solution components and imposing various length perturbations on the fiber, we measure muscle properties such as power, force, velocity, stiffness, and rate constants of the myosin cross-bridge cycle. We primarily test the mechanical properties of indirect flight and jump muscles from our transgenic Drosophila, but recently have begun evaluating cultured myotubes and tendons grown from muscle cell lines and fibroblasts.
We evaluate muscle mechanical properties using the skinned fiber technique. The mechanics apparatus includes a pair of hooks, one attached to a force transducer and the other to a high-speed length motor. The muscle fiber is mounted onto the hooks and lowered into a solution chamber. By varying the concentrations of the solution components and imposing various length perturbations on the fiber, we measure muscle properties such as power, force, velocity, stiffness, and rate constants of the myosin cross-bridge cycle. We primarily test the mechanical properties of indirect flight and jump muscles from our transgenic Drosophila, but recently have begun evaluating cultured myotubes and tendons grown from muscle cell lines and fibroblasts.
In collaboration with the Cammarato laboratory, at Johns Hopkins University, we have developed a method to isolated myofibrils from Drosophila Jump muscle. We can now measure additional mechanical parameters such as sarcomere activation and relaxation rates. Click the link below to see a video of a ~ 2 micrometer diameter jump muscle myofibril contracting on their specialized myofibril apparatus.
Muscle Dissection
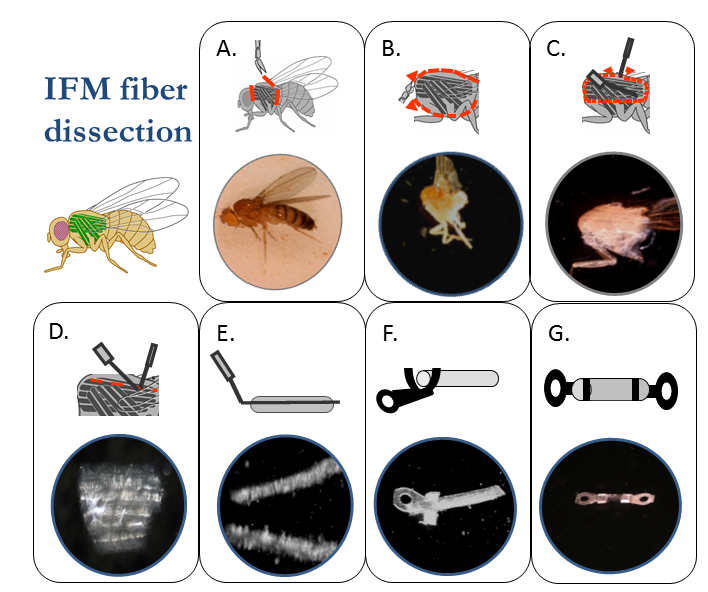
Indirect Flight muscle preparation: The steps of preparing an IFM fiber for mechanical measurements are A) isolation of the thorax by removing the head, abdomen and wings, B) splitting the thorax in half and immersing it in skinning solution, C) removing the bundle of DLM fibers, D) separating the 6 fibers in the bundle from each other, E) splitting one of the fibers in half, F) clamping the tabs of the T-clips around the ends of the fiber to produce G) a clipped IFM fiber preparation.
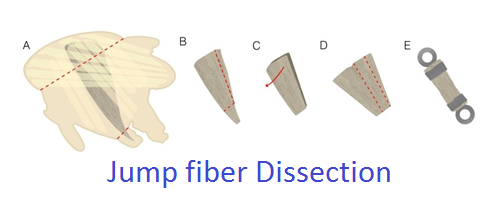 Jump muscle preparation: After isolating the thorax, the jump muscle is A) removed from the half thorax by cutting as shown by the red dotted lines. B) The jump muscle is pared down in size and C) the two-layered muscle is split open to become one single layer of fibers. D) A strip of 8-10 fibers is cut from the muscle and E) fastened between two T-clips.
Jump muscle preparation: After isolating the thorax, the jump muscle is A) removed from the half thorax by cutting as shown by the red dotted lines. B) The jump muscle is pared down in size and C) the two-layered muscle is split open to become one single layer of fibers. D) A strip of 8-10 fibers is cut from the muscle and E) fastened between two T-clips.
Click on the following link to see a dissected jump muscle contracting as we add calcium:
Locomotion Assays
These assays are fun and fast ways to characterize the basic locomotion properties of our mutant fly lines. Assays include flight ability, wing beat frequency, wing stroke amplitude, and jump ability. This analysis reveals the influence of changes in muscle mechanical performance on locomotion, sometimes in unexpected ways.
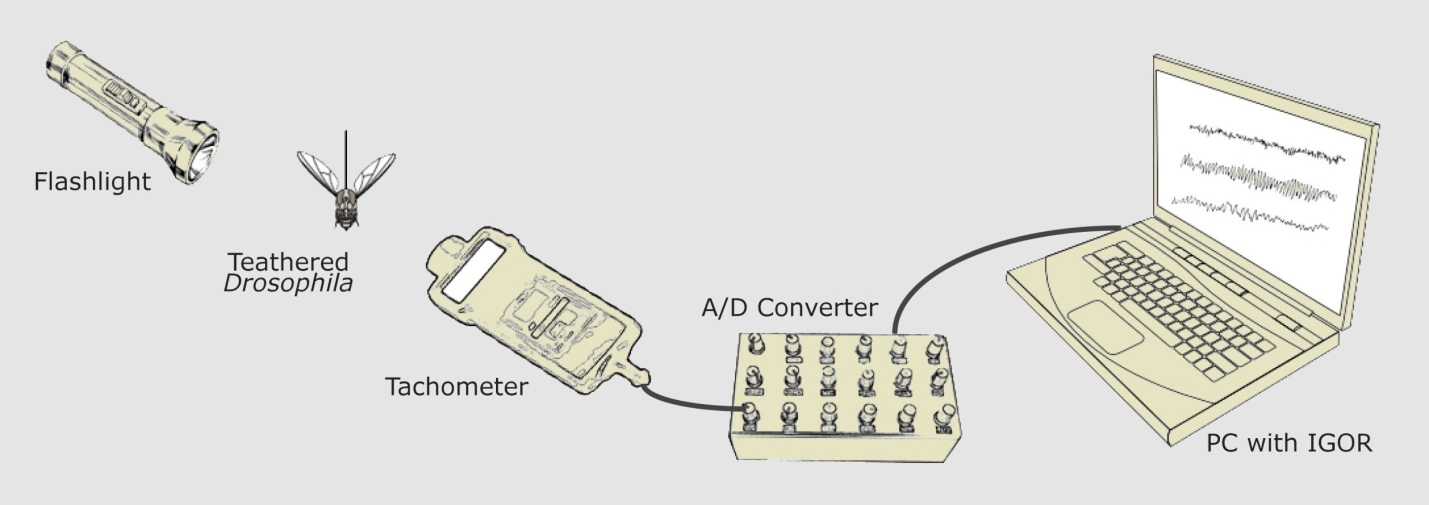
Wing Beat Frequency: The wings of the fruit fly interrupt the flow of light from the flashlight to the tachometer. Using a Fourier Transform, a computer program anaylzes the frequency of variation in light intensity, calculating the beats per second of a fruit fly's wings.
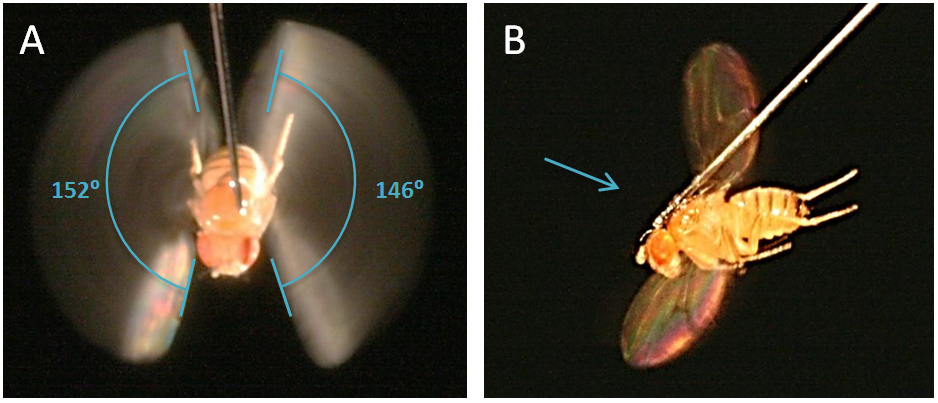
Wing Stroke Amplitude: A) Sample image showing how wing stroke angle is measured. B) Side view of fruit fly. The blue arrow shows the alignment of the camera lens with respect to the fly. The camera is lined up perpendicular to the wing path, not the parallel to the long axis of the fly's body.
In vitro Motility Assays
We characterize actin and myosin interactions in the absence of any other muscle proteins in the in vitro motility assay. Myosin is purified from our fruit flies and then adhered to a glass coverslip flow cell. Fluorescently-labeled actin filaments are moved by the myosin heads, powered by MgATP hydrolysis (see video clip). We measure the actin velocity to determine the influence of mutations on myosin performance.
http://www.youtube.com/embed/JpHFezFhPRo