How do mutations in muscle proteins cause muscle and heart diseases?
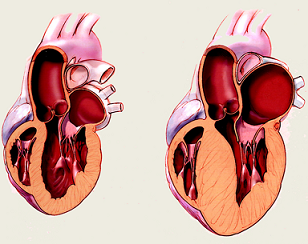
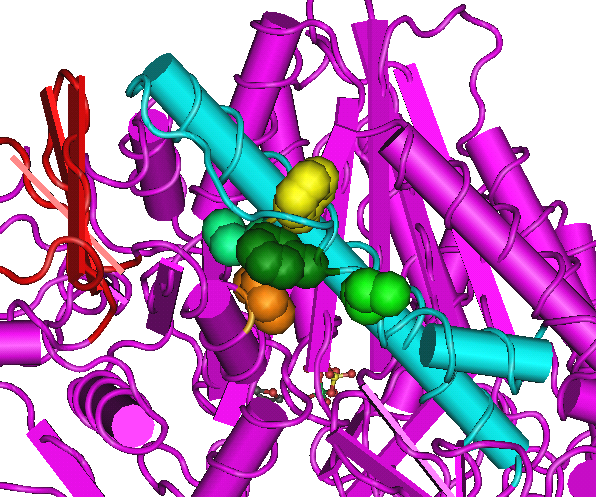 On the left is a healthy heart. On the right is a heart with excessive muscle mass that pumps an inadequate amount of blood. Very little is known about how genetic mutations alter muscle structure leading to various myopathies. In heart muscle, at least 200 different point mutations in myosin cause familial hypertrophic cardiomyopathy (FHC), the leading cause of sudden cardiac death among young adults. There is a critical need to determine the affects FHC mutations have on muscle mechanical properties. This research will enable more specific treatment options for FHC patients. The lower figure shows the location of three point mutations (shades of green) in myosin that cause FHC.
On the left is a healthy heart. On the right is a heart with excessive muscle mass that pumps an inadequate amount of blood. Very little is known about how genetic mutations alter muscle structure leading to various myopathies. In heart muscle, at least 200 different point mutations in myosin cause familial hypertrophic cardiomyopathy (FHC), the leading cause of sudden cardiac death among young adults. There is a critical need to determine the affects FHC mutations have on muscle mechanical properties. This research will enable more specific treatment options for FHC patients. The lower figure shows the location of three point mutations (shades of green) in myosin that cause FHC.
How is mechanical variation between muscle fiber types achieved?
Human muscles are composed of three fiber types: Type I slow, Type IIa fast and type 11b very fast. Slow fibers are used for low force, high speed movements; fast for high speed, high force movements. The upper figure shows sections of different muscle types, distinguishable by different colors.
Drosophila flight muscle (IFM), which contracts 200 times per second, is the fastest known muscle type (above). Comparing the properties and proteins of this muscle type with much slower muscle types (below) reveal mechanisms by which mechanical muscle properties are determined. Determining the mechanisms by which different muscle types produce various mechanical outputs will be important in the future development of lab-engineered muscles.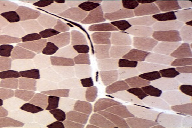

How do motor proteins convert chemical energy to force and motion?
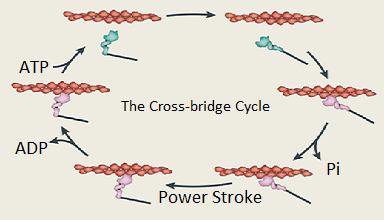 Myosin motors cyclically bind to actin filaments and undergo a conformational change called the power stroke. This causes a muscle to shorten. The cycle is fueled by ATP. Understanding details of the conversion of chemical energy to force and motion is critical to understanding how mutations in muscle proteins cause muscle and heart diseases.
Myosin motors cyclically bind to actin filaments and undergo a conformational change called the power stroke. This causes a muscle to shorten. The cycle is fueled by ATP. Understanding details of the conversion of chemical energy to force and motion is critical to understanding how mutations in muscle proteins cause muscle and heart diseases.